Students and Teachers Forum
The atmosphere of the earth is divided into five layers. These layers are shown in the picture .....
Air is a mixture because it contains many types of gases and water vapours so is considered as a mixture Similarly soil contains many types of organisms ,humus and different types of rock particles .....
You might have used a tea strainer at your home, which is used to separate tea leaves from water. Another example is Filters, they are very fine sieves. So Sieving is used to separate large particles from suspensions, usually .....
Magnetic separation is based on the differing degrees of attraction exerted on various minerals by magnetic fields. Magnetic separation is a process in which magnetically susceptible material is extracted from a mixture using a magnetic .....
How does your mother separate any stones or small pieces of impurities from rice? Yes, simply picking up by her hand. That is hand picking. This process can be used to separate impurities that are visible by our naked .....
Take a mixture of sand and water. How can you separate them? Yes, by filtration you can separate it. Take a filter paper and a beaker. Pour the mixture through the filter paper. You can obtain sand on the filter paper and water in the .....
Let us take an example of a suspension would be sand in water. The suspended particles are visible under a microscope and will settle over time if left undisturbed. So, a suspension is a heterogeneous mixture containing solid .....
Some characteristics: - They have non-uniform(different) composition of particles. - The components are visible to eyes. - They can be easily separated. - Each component shows its original .....
Look at the above picture. You must be familiar with all the items. Lets take can example of dairy milk. Have many ingredients did you see? You can only see one. Because in this case, many ingredients are mixed together and we cannot .....
However, they may reflect light from other sources. These objects are called non-luminous .....
Look at the figure above. What did you see? It is a burning candle emitting light. Luminious objects are those which emits lights of their own.
Other examples are the sun, the bulb, star etc. Any object is considered luminous if it .....
These substances never dissolve in water or any other solvent at room temperature and pressure. Sugars and inorganic salts are also examples of insoluble substances. Insoluble substances cannot be extracted from a .....
Before we dive into solutions,
.....
Take some sugar and put it in a glass of water. Stir it with a spoon. Does the sugar dissolve in water? Yes, sugar dissolves in water. Like sugar, salt also dissolves in water. Sugar and salt are soluble .....
Examples: - Wood - LPG - Coal - .....
In the above picture, light can not pass through the apple. So apple is an opaque object. Examples: - Book - Metal - Gold - .....
You can not see through the walls. It is because, the light can not pass through the walls. But, the light passes easily through the glass of window. So, you can see through the window .....
Matter can exist in one of three main states: solid, liquid, or gas. Solid matter is composed of tightly packed particles. A solid will retain its shape; the particles are not free to move around. Liquid matter is made of more loosely packed .....
There are many types of physical properties. Commonly used examples include density, color, odor, hardness, and volume. Physical properties are further classified based on whether they are extensive or .....
Matter is anything, such as a solid, liquid or gas, that has weight (mass) and occupies space. For anything to occupy space, it must have volume. Thinking .....
Knowing the boiling point of a substance provides information to determine what the substance is if its unknown. If you are making a substance, checking the boiling point will let you know if you have made what you set out to make, (because .....
A good example is you would never build a house out of ice to live in if you live in a hot place like Africa. Ice has a melting point of 0o C and that isn't a common temperature you would find in most places and therefore the 'house' .....
As you know, ice melts into water on heating. The water evaporates into the vapour on further heating. Likewise, most of the solids melt into liquid and then evaporate into the vapour state on heating. But, some solids such as .....
Sublimation can be important during the recovery of compounds that are suspended or dissolved in a fluid or a solid such as dry ice. Sublimation is the transition of a substance from a solid to a gas without passing through the liquid .....
The temperature at which a material changes from a gas to a liquid; the same as the boiling point is condensation point. For eg: the condensation point of water is 100oC (same as its boiling .....
Have you ever seen water boiling? Look at the figure above. What you see? You can see vapours in large amount. How it is possible? Simply when the water is heated. Thus, evaporation is the process, in which substance in a liquid state .....
Have you noticed in your frize, how water becomes ice? If tempature inside the frize is around 00C, water frizes in to ice. So, The freezing point of liquid is the temperature at which it changes phase from a liquid to a .....
Take a glass of water and boil it in a vessel. What happens when it reaches its boiling point? It starts evaporating. This is known as .....
Yes, the ice melts into the water at 0°C. 0°C is the melting point of ice or water. Melting point of a substance is the temperature at which it changes its state from solid to .....
You might have eaten 'kulfi'. A 'kulfi' is made by freezing water and other flavours. Freezing can be defined as a process of converting liquid into solid by decreasing the temperature. As temperature decreases, the disorganized .....
Look at the figure below. Here, the ice is in the process of converting to .....
There are 3 states of matter. They are: Solid Liquid Gas One state can be changed to another state and vice versa. For eg: lets take water. Water can be in 3 states. 1) Ice (solid) 2) Liquid water (liquid) 3) Steam .....
3. Physical change results in change in states of a substance. E.g. A hot molten iron when left to cool form a solid steel. 4. Physical change results in change in the shape of an object. E.g. When a steel is .....
So, the change in Physical appearance of substance without a change in its chemicalcomposition is called physical change. Other examples are: - hammering the steel - Dissolving sugar in water - Breaking glass .....
You may have burnt wood to make fire. After the fire has completely burnt out, what is left behind? Ashes! Ash is a new substance formed due to the burning of another substance, wood. This is a chemical change. So, the change in the .....
The matter may be in the form of solid, liquid or gas. A stone is solid, occupies space and has mass. The gas inside the balloon occupies space and has mass. A glass of water occupies space and has certain mass. Thus, we feel the presence of .....
I live in Sainbu Bhaisepati. I am from Lalitpur. Where are you .....
xy2xy33×xy-2yx-32 Now using rule, x-m=1xm , we get, xy2xy33×xy-2xy32 Use rule, (xy)m=xm ym xy2×3xy3×3 × xy-2×2xy3×2 xy6xy9 × xy-4xy6 Now use rule,xm.
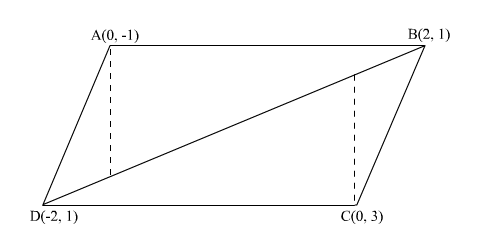
Here we have given vertices of a quadrilateral ABCD. Let A( 0 , -1) = ( x1 , y1 ) B(2,1)= ( x2 , y2 ) C ( 0,3)= ( x3 , y3) D ( -2, 1) = (x4 , y4 ) We know area of qd.ABCD = 1 / 2 [ x1y2 - y1x2 + .....
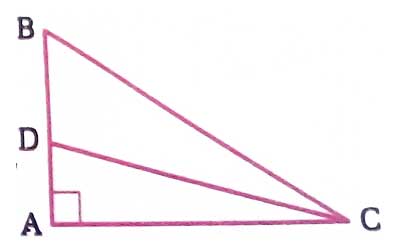
Take triangle ABC, BC2 = AB2 + AC2 or, BC2 - AB2 =AC2 ..................i) Take triangle ADC, DC 2 + AC2= AD2 or, AC2 = DC2 - .....
As in your previous question, Take triangle ABC, BC2 = AB2 + AC2 or, BC2 - AB2 =AC2 ..................i) Take triangle ADC, DC 2 + AC2= AD2 or, AC2 = DC2 - .....
Here to add them you can simply let a = cos2θ Then it becomes, 4cos2θ + 5 cos2θ + 9cos2θ = 4a + 5a + 9a = 18 a finally put a .....
'i' is used as a loop counter in programming. For example: If i = 1 then i ++ = 1 + 1 =2= 2 ++ is used for increment and i counts the number of times it incremented -- is used for decrement. i-- counts .....
It was dicovered by Joseph Priestley. Joseph Priestley (1733-1804) discovered Oxygen gas on 1 August 1774 in the laboratory at Bowood House, Wiltshire, England; seat of the Marquess of .....
The country’s dependence on India for imports soared to 58.06 percent in the 2000s. You may consider this dependency on India to lack of development of productivity and competency compared to Indian products. You may look for the market .....
We have given volume of can = V = 308 liter = 308 x 1000m3 Area of base = A = 154cm2 Now we know volume = A X height or, 308 x 1000m3= 154cm2 X height Find Height, Again A = π r2 or, 154 cm2 = π .....
To factorise it we can complete square method. So, x 4+ y4 / 4 = ( x2 )2 + (y2/2)2 + 2. x2 .y2 /2- 2 .x2 .y2/2 Now use a2 + b2 + 2ab = (a +b )2 we get; = ( x2 + .....