Students and Teachers Forum
Development is an activity that brings bout the positive change to the individual, community and to the nation. Any activities that do not bring about any positive changes cannot be termed as development like change in .....
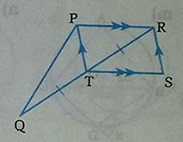
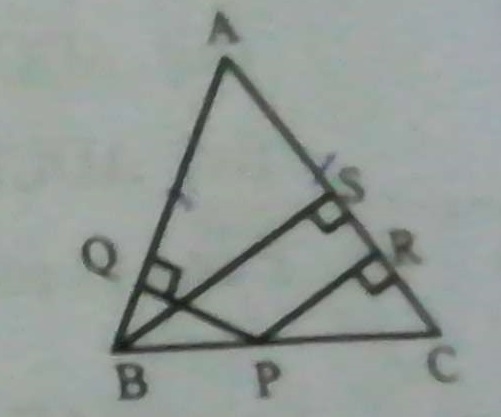
1. Area of ∆PTR = Area of ∆RTS [ Since Diagonal RT bisect the parallelogram] 2. Area of ∆PQT= Area of ∆RTS[ Since median PT bisect ∆PQR] 3. Area of ∆PQT = Area of ∆RTS [ From 1 and .....
People of diverse religion and belief are the dominant features of Nepali society. People have a sense of unity, though living in the diversity. Mostly, Brahmins and Chhetris are regarded as Hindu believers. But people from the Mongoloid .....
Final velocity (v) = 90km/hour = 90000/3600 .....
The social costumes differ from one place to another because of the following things: The social norms and values differ because of the geographical distance. The social costumes of Himalayan region is different than of Hilly and Terai. This is .....
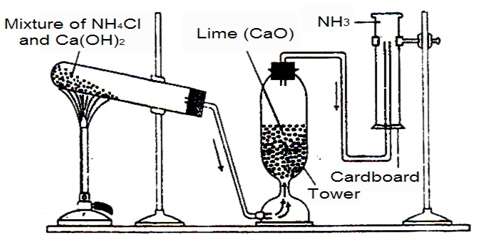
a. The balanced chemical equation of the process of the gas being prepared (i.e. ammonia) is given below: 2NH4Cl + Ca(OH)2 → 2NH3 +CaCl2 + 2H2O b. The mouth of the hard glass test tube is directed downwards .....
When ammonium chloride (NH4Cl) and calcium hydroxide ( Ca(OH)2 ) are heated together, ammonia (NH3) gas is produced. The balanced chemical equation is given below: 2NH4Cl + Ca(OH)2 → 2NH3 +CaCl2 + .....
When carbon dioxide is passed through water under pressure, it product will be Carbonic acid(H2CO3). CO2 + .....
When carbon dioxide (CO2) is heated with red hot coke at 9000 C, it produce carbon monoxide. CO2 + C → .....
Calcium carbonate(CaCO3) is strongly heated until it undergoes thermal decomposition to form calciumoxide(CaO) and carbon dioxide(CO2). Thermal decomposition; CaCO3(s) → CaO(s) + .....
When carbon dioxide gas is passed through lime water for a long time, then the white precipitate of CaCO3 which is formed initially dissolves. This is due to formation of a soluble salt calcium hydrogen carbonate ( .....
Uses of carbondioxide are as follow. i) Plants use carbon dioxide to produce carbohydrates (sugars and starches) in the process known as photosynthesis. ii) Carbon dioxide in solid (dry ice) and in liquid form is .....
If limestone(CaCO3) is heated strongly, it breaks down to form calcium oxide(CaO) and carbon dioxide(CO2). Calcium oxide is also called quicklime. It is yellow when hot, but white when .....
When a hydrocarbon (methane - CH4) is burnt in air or oxygen, a large amount of heat is produced along with carbon dioxide and water. A balanced chemical equation is: CH4 + 2O2 ----------> CO2 + .....
Carbon react with oxygen present in air to form carbon dioxide. C + O → .....
The Haber's process is described in the figure .....
Ammonia is used for i) Ammonia is also used as a refrigerant gas, ii) For purification of water supplies. iii) For the manufacture of plastics, explosives, textiles, pesticides, dyes and other .....
It is an acidic oxide of carbon. A solution of the gas (CO2) in water is called carbonic acid(H2CO3). It is a weak acid. Carbon dioxide also reacts with alkalis like sodium hydroxide forming salts, called carbonates and .....
Laboratory Preparation of Ammonia Gas Principle : Ammonia Gas is prepared in the laboratory by heating a mixture of ammonium chloride ( NH4Cl ) and calcium hydroxide or slaked lime [Ca(OH)2]. 2NH4Cl+CaOH2→CaCl2+2H2O+2NH3
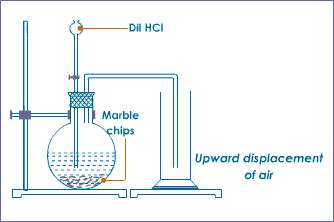
Principle : Carbondioxide gas is prepared in laboratory by the action of dilute hydrochloric acid on calcium carbonate( limestone or marble). The reaction which takes place is : CaCO3+ 2HCL →CaCl2+ H2O+CO2 . fig: Lab preparation of .....
Ammonia is usually prepared by heating a mixture ofammonium chloride and slaked lime in the ratio of 2 : 3 by .....
Joseph Black (16 April 1728 – 6 December 1799) was a Scottish physician and chemist, known for his discoveries of magnesium, latent heat, specific heat, and carbon .....
When a burning magnesium ribbon is introduced in the jar containing the carbon dio-oxide, it produces the black and white smoke. The balanced chemical reaction is shown below: Mg + CO2→ MgO + .....
a.) The balanced chemical equation is : CaCO3+ 2HCL →CaCl2+ H2O+CO2 b.) Carbon dioxide is a heavy gas. It is heavier than air so instead of going up it goes down. So the mouth of the jar is directed upward and this method of collection .....
White blood cells (WBCs), also called leukocytes or leucocytes, are the cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. The three major types of white blood cells .....
Here, For first case, The price of commodity = Rs. 10,000 - D% of Rs. 10,000 = Rs. .....
For first camera, Cost price ( C.P1 ) = a Selling price ( S.P1 ) = a + 20% of a or, 24000 = a + 0.2 a or, 1.2 a = 24000 ∴ a = Rs. 20,000 For second camera, Cost price ( C.P2 ) = b Selling price ( S.P2 ) = b - 20% of .....
Given, Price of the article with VAT = Rs 1356 VAT percent on the article = 13% Let the price of the article without VAT be x. Then, Price of the article with VAT = x + 13% of x 1356 = x + ( 13/100 ) * x 1356 = x + 0.13x 1.13x = 1356 x = Rs .....
Join C, O and D, O. 1. <OCA = <ODP = 90 [ Line joining center to the mid-point of the chord is perpendicular to chord] 2. OC = OD [ Equal chords are equidistant from the center] 3. <OCD = < ODC [ In triangle ODC, OC = AD, isosceles .....
Join P and Q. 1. < BAP = < PRD [ Being APRB is cyclic quadrilateral] 2. < PRD + PCD = 180 [ Being opposite angles of cyclic quadrilateral PRDC] 3. <BAP + < PCD = 180 [ From 1 and 2] 4. AB // CD [ <BAP + < PCD = 180, .....
Join R and B, B and S, A and B. 1. < AQS + < ABS = 180 [ Being opposite angle of cyclic quadrilateral ABSQ] 2. < RPA + < ABR = 180 [ Being opposite angle of cyclic quadrilateral APRB] 3. <RPA + <AQS = 180 [ Being .....
Join PQ, 1. < PCB = < PQA [ Being PQBC is cyclic quadrilateral] 2. < ADP + <PQA = 180 [ Being opposite angles cyclic quadrilateral] 3. < ADP + < PCB = 180 [ From 1 and .....
Let, 1 sterling = Rs. 100 Now, Rs. 600000 = 600000 / 100 = 6000 Sterling After one week, due to dealuation of the Nepalese rupees, exchange rate becomes, 1 sterling = Rs. 100 + 5% of Rs. 100 = Rs. 105 Now, 6000 sterling = .....
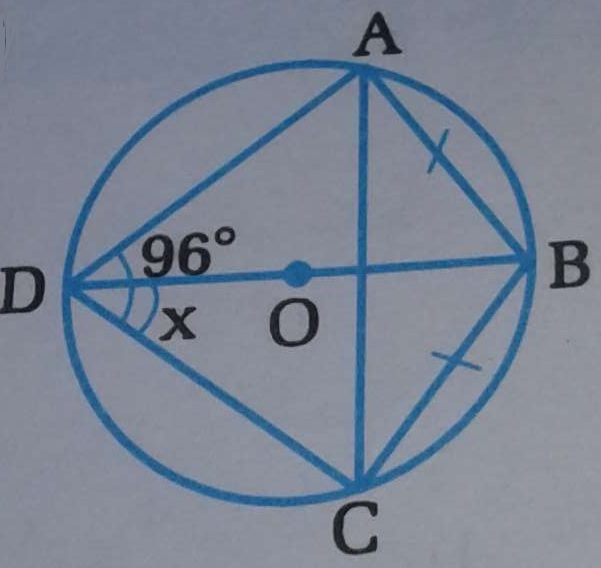
∠Here, ABCD is cyclic quadrilateral. ∠ XAC= ∠ ACX [ XA =XC, radii of same circle] ∠ ACX= ∠ ABD [ Cyclic quadrilateral] ∠ XAC= ∠ CDB [ Cyclic quadrilateral] Hence, ∠ABD = ∠ .....
Construct : Join C and D 1. < BAC = < CDE [ Being ABCD is the cyclic quadrilateral] 2. < CDE = < CFE [ Inscribe angle on the same arc CE 3. < BAC = < CFE [ From 1 and 2] 4. AB // EF .....
Here, MP = SP = Rs. 1200 [ Since no discount ] Now, SP with VAT = SP + VAT % of SP = Rs. 1200 + 13% ⨉ Rs. 1200 .....
Given, Price of the good = Rs 52100 VAT percent on the good = 15% Using formula, VAT amount on the good = VAT% of price of the good = 15% of 52100 = ( 15/100 )* 52100 = Rs 7815 Hence, the VAT amount on the purchase of the good is Rs .....
.jpg)
.jpg)